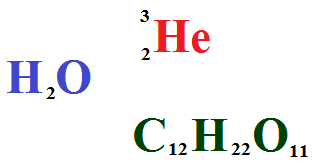A chemical reaction can be classified either according to its molecularity or according to its order. The order is a description of the number of concentration terms multiplied together in the rat equation. Order is the number of concentration factors in the rate equation. The order of a reaction can only be found by experiment and cannot be worked out from the equation of the reaction.
“A reaction whose rate depends upon the second power of the concentration of the reactant is called second order reaction.”
In the second order reaction
A + B --> Products
The rate equation is
Rate = - d[A]/dt = - d[B]/dt = k[A][B] or rate(for one type of reactants) = [A]^2
For the second order reaction, the rate of reaction increases with the concentration of reactants. In second order reaction the rate of reaction is proportional to the concentration of reactant. When a reaction is second order in a reactant, the doubling its concentration increases rate by a factor 22 and tripling the concentration increases rate by 32 and so forth.
The rate of a reaction depends on concentration but rate constant is independent of concentration. The rate as well as the rate constant are affected by temperature and catalyst.
For example, saponification of ethyl acetate, the chemical equation for this reaction is
CH_3COOC_2H_5 + NaOH --> CH_3COONa + C_2H_5OH
The molecules (CH_3COOC_2H_5 + NaOH) are involved in this reaction and hence the molecularity of the reaction is two. This rate of this reaction has been found to obey the following rate.
-d[CH_3COOC_2H_5]/dt a [CH_3COOC_2H_5][NaOH]
Hence the order to this reaction is two.
“A reaction whose rate depends upon the second power of the concentration of the reactant is called second order reaction.”
In the second order reaction
A + B --> Products
The rate equation is
Rate = - d[A]/dt = - d[B]/dt = k[A][B] or rate(for one type of reactants) = [A]^2
For the second order reaction, the rate of reaction increases with the concentration of reactants. In second order reaction the rate of reaction is proportional to the concentration of reactant. When a reaction is second order in a reactant, the doubling its concentration increases rate by a factor 22 and tripling the concentration increases rate by 32 and so forth.
The rate of a reaction depends on concentration but rate constant is independent of concentration. The rate as well as the rate constant are affected by temperature and catalyst.
For example, saponification of ethyl acetate, the chemical equation for this reaction is
CH_3COOC_2H_5 + NaOH --> CH_3COONa + C_2H_5OH
The molecules (CH_3COOC_2H_5 + NaOH) are involved in this reaction and hence the molecularity of the reaction is two. This rate of this reaction has been found to obey the following rate.
-d[CH_3COOC_2H_5]/dt a [CH_3COOC_2H_5][NaOH]
Hence the order to this reaction is two.