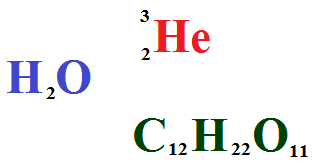Solutions are homogeneous mixtures of two or more than two components. By homogeneous mixture it is meant that composition and properties are uniform throughout the mixture. Components of solution are called solute and solvent. Every solution is made up of a solvent and one or more solutes.
If water is the solvent, the solutions are called aqueous solutions and solutions in which other solvents like benzene, ether etc are used are called non-aqueous solutions. A solution containing one solute dissolved in a solvent is called a binary solution that is consisting of two components. Here each component may be solid, liquid or in gaseous state which is summarized in the table below.
Most chemical reactions are carried out in solutions. Body fluids are also solutions of various components in water. The most common types of solutions used in chemistry labs are solid-liquid, liquid-liquid and gas-liquid solutions. Thus a solution plays an integral part of lives.
If water is the solvent, the solutions are called aqueous solutions and solutions in which other solvents like benzene, ether etc are used are called non-aqueous solutions. A solution containing one solute dissolved in a solvent is called a binary solution that is consisting of two components. Here each component may be solid, liquid or in gaseous state which is summarized in the table below.
Most chemical reactions are carried out in solutions. Body fluids are also solutions of various components in water. The most common types of solutions used in chemistry labs are solid-liquid, liquid-liquid and gas-liquid solutions. Thus a solution plays an integral part of lives.